Share this
Myth: Hydrogen Gas Leaks are More Hazardous Than Gasoline Leaks
by Derek Green on Wed, Apr 24, 2024 @ 06:04 AM
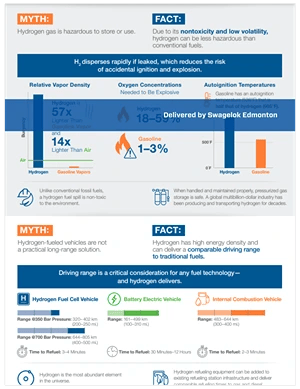
Hydrogen, being nontoxic and less volatile, can pose fewer hazards than conventional fuels. In this article we look at relative vapor density, oxygen concentrations, and autoignition temps; offer a new infographic; and invite you to download details on our hydrogen-specific products and services.
There is a common misperception that hydrogen gas storage is inherently riskier than storage of oil or gasoline. But when handled and maintained properly, pressurized gas storage is safe. Let's look at three factors to consider:
1. Relative vapor density
Hydrogen, being 14 times lighter than air and 57 times lighter than gasoline vapor, disperses quickly into the atmosphere when leaked. This rapid dispersion reduces the risk of localized explosive concentrations forming, making immediate ignition less likely compared to heavier gases that linger near their leakage points. On the other hand, its low density allows hydrogen to spread over a larger area, potentially reaching ignition sources far from the leak's origin.
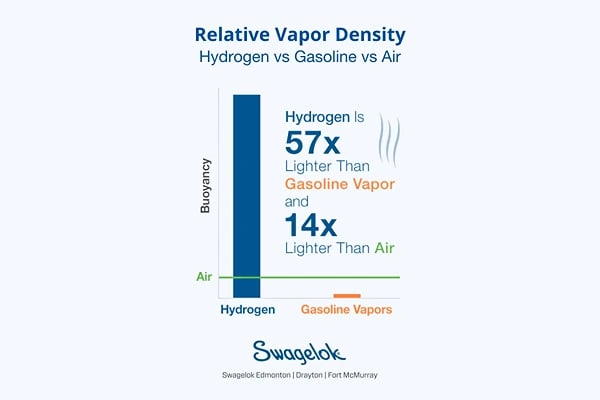
Safely storing hydrogen requires understanding its tendency to rise and accumulate in enclosed spaces, risking explosive mixtures without adequate ventilation. Conversely, gasoline vapor, being heavier than air, pools at ground level, creating different hazards that need containment to avoid accumulation. Designing storage facilities thus demands a thorough grasp of these gases' behaviors to customize ventilation, leak detection, and containment strategies to their specific properties.
2. Oxygen concentrations
Hydrogen disperses quickly when leaked due to its low density, significantly reducing the risk of accidental ignition and explosion compared to gasoline. This rapid dispersion minimizes the formation of explosive concentrations near the leak source, unlike gasoline vapor, which, being heavier than air, accumulates at ground level, increasing the risk of ignition. The inherent property of hydrogen to quickly mix with air and dilute reduces the likelihood of localized flammable conditions, making it inherently safer in the context of leak scenarios.

3. Autoignition temperatures
Gasoline has an autoignition temperature of 536°F, significantly lower than hydrogen's 995°F. This higher autoignition threshold makes hydrogen storage inherently safer, as hydrogen requires much higher temperatures to ignite spontaneously compared to gasoline.
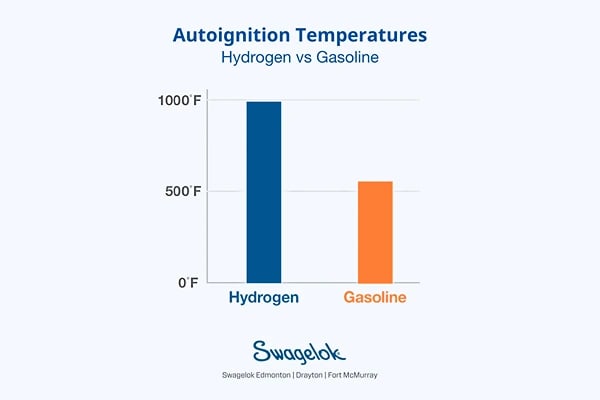
Hydrogen's higher autoignition temperature reduces the likelihood of accidental ignitions in scenarios where external heat sources might be present, offering an additional safety margin in the storage and handling of hydrogen.
Members of the public may view hydrogen gas leaks as more dangerous than conventional fossil fuel leaks due to hydrogen's association with high-profile accidents and its characteristics like flammability and invisibility when burning. This perception doesn't square with the extensive safety measures and technological advancements that have been developed to mitigate risks, along with hydrogen's successful safety record in industrial applications.
Hydrogen myths infographic (click image for full size)
Learn more and download resources
View our website page about products and services for the hydrogen industry. It covers hydrogen-compatible Swagelok products, fluid system assemblies for hydrogen applications, and advice we can offer on fluids issues in electrolyzers, micro-grids, fuel cells, virtual pipelines, and more.
Related articles
Share this
- Local Services (103)
- Field Advisors (101)
- Training & Events (86)
- Fittings (81)
- Valves (67)
- Resources (62)
- Tubing (62)
- Sampling Systems (60)
- Design & Assembly (57)
- Resources - Downloads (41)
- Hose & Flexible Tubing (39)
- Frequently Asked Questions (37)
- Regulators (34)
- Cost Savings (33)
- Oil & Gas (33)
- Videos (33)
- Steam Systems (29)
- Mechanical Seal Support (17)
- Measurement Devices (15)
- Gas Distribution Systems (9)
- Rentals (6)
- Safety (6)
- winterization (6)
- Covid (3)
- Hydrogen & Clean Energy (3)
- About Us (1)
- April 2024 (4)
- March 2024 (2)
- January 2024 (3)
- December 2023 (2)
- November 2023 (3)
- October 2023 (2)
- September 2023 (3)
- August 2023 (3)
- July 2023 (3)
- June 2023 (2)
- May 2023 (4)
- April 2023 (2)
- March 2023 (2)
- February 2023 (3)
- January 2023 (2)
- December 2022 (1)
- November 2022 (1)
- October 2022 (2)
- September 2022 (5)
- August 2022 (3)
- July 2022 (6)
- June 2022 (4)
- May 2022 (3)
- April 2022 (1)
- March 2022 (2)
- February 2022 (3)
- January 2022 (4)
- December 2021 (4)
- November 2021 (6)
- October 2021 (3)
- September 2021 (5)
- August 2021 (9)
- July 2021 (5)
- June 2021 (7)
- May 2021 (7)
- April 2021 (4)
- March 2021 (3)
- February 2021 (3)
- January 2021 (2)
- December 2020 (3)
- November 2020 (3)
- October 2020 (2)
- September 2020 (3)
- August 2020 (3)
- July 2020 (3)
- June 2020 (3)
- May 2020 (3)
- April 2020 (2)
- March 2020 (3)
- February 2020 (3)
- January 2020 (3)
- December 2019 (2)
- November 2019 (3)
- October 2019 (3)
- September 2019 (2)
- August 2019 (3)
- July 2019 (2)
- June 2019 (2)
- May 2019 (3)
- April 2019 (3)
- March 2019 (3)
- February 2019 (2)
- January 2019 (3)
- December 2018 (2)
- November 2018 (2)
- October 2018 (4)
- September 2018 (2)
- August 2018 (3)
- July 2018 (3)
- June 2018 (2)
- May 2018 (4)
- April 2018 (3)
- March 2018 (3)
- February 2018 (3)
- January 2018 (4)
- December 2017 (1)
- November 2017 (4)
- October 2017 (4)
- September 2017 (4)
- August 2017 (5)
- July 2017 (3)
- June 2017 (4)
- May 2017 (4)
- April 2017 (3)
- March 2017 (5)
- February 2017 (4)
- January 2017 (4)
- December 2016 (3)
- November 2016 (3)
- October 2016 (4)
- September 2016 (3)
- August 2016 (4)
- July 2016 (2)
- June 2016 (2)
- May 2016 (2)
- April 2016 (4)
- March 2016 (2)
- February 2016 (3)
- January 2016 (4)
- December 2015 (4)
- November 2015 (4)
- October 2015 (5)
- September 2015 (2)
- August 2015 (4)
- July 2015 (5)
- June 2015 (2)
- May 2015 (3)
- April 2015 (5)
- March 2015 (3)
- February 2015 (4)
- January 2015 (3)
- December 2014 (5)
- November 2014 (4)
- October 2014 (4)
- September 2014 (4)
- August 2014 (4)
- July 2014 (5)
- June 2014 (4)
- May 2014 (4)
- April 2014 (5)
- March 2014 (4)
- February 2014 (4)
- January 2014 (4)
- December 2013 (3)
- November 2013 (4)
- October 2013 (5)
- September 2013 (4)
- August 2013 (5)
- July 2013 (4)
- June 2013 (3)
- May 2013 (4)
- April 2013 (5)
- March 2013 (2)
- February 2013 (3)
- January 2013 (5)
- December 2012 (3)
- November 2012 (3)
- October 2012 (5)
- September 2012 (3)
- August 2012 (4)
- July 2012 (4)
- June 2012 (1)